Our publications keep professionals working across the public, private, and academic sectors informed on the most important developments and issues in health security and biosecurity.
Find an article or report by keywords:
UPMC Center for Health Security, 2014
Smallpox is considered one of the most serious bioterrorist threats. It was used as a biological weapon during the French and Indian Wars, (1754 to 1767) when British soldiers distributed smallpox-infected blankets to American Indians. In the 1980s, the Soviet Union developed variola as an aerosol biological weapon and produced tons of virus-laden material intended for release via intercontinental ballistic missiles.
Unless otherwise noted, all information presented in this article is derived from the following 2 sources:
Smallpox is a highly contagious and deadly disease caused by the variola virus. It was estimated to have infected 300 million people in the 20th Century before it became the only human infectious disease ever to be completely eradicated. Following a global vaccination campaign led by the World Health Organization (WHO) (the Intensified Global Eradication Program), the last naturally occurring case of smallpox was in Ethiopia in 1977. The World Health Assembly declared smallpox eradicated in 1980 and recommended that all countries cease routine smallpox vaccination.
After the eradication of smallpox, the WHO recommended that all remaining specimens of variola be destroyed or sent to 1 of 2 designated high containment reference laboratories located in the U.S. and Russia. Today, the only potential source of smallpox infection is an unintentional laboratory release or a biological attack.
Smallpox is considered one of the most serious bioterrorist threats. It was used as a biological weapon during the French and Indian Wars, (1754 to 1767) when British soldiers distributed smallpox-infected blankets to American Indians. In the 1980s, the Soviet Union developed variola as an aerosol biological weapon and produced tons of virus-laden material annually intended for intercontinental ballistic missiles.
Several factors contribute to the concern about the use of smallpox as a biological weapon:
Variola can spread from person to person.
There is no widely available or licensed treatment for the disease.
It has a high fatality rate.
Since routine smallpox immunization ceased in the United States in 1972 and in all other countries by 1983, the global population is extremely vulnerable to the disease. Most of the world's population has never been vaccinated or was vaccinated so long ago that immunity to smallpox has waned.
Variola is relatively stable as an aerosol.
The infectious dose is small.
Because there are no symptoms at the time of exposure, a covert release of variola may not be detected until sick people begin showing up at doctor's offices and hospitals. (See "The History of Bioterrorism: Smallpox," a short video from the CDC.)
Smallpox spreads from person to person by respiratory droplets or direct contact with body fluids. Contaminated clothing or bedding may also transmit the virus. Animals and insects are not known to carry or spread the virus to humans. The incubation period for smallpox is 7 to 17 days.
Individuals diagnosed with or suspected of having smallpox should be isolated to prevent the spread of disease. Household and other face-to-face contacts of patients who have, or are suspected of having, smallpox should be vaccinated and placed under surveillance.
For smallpox patients in the hospital, airborne precautions (see CDC Isolation Precautions Guidelines) should be followed, and all hospital personnel should be vaccinated.
Diagnosis of smallpox is based on clinical presentation of symptoms in the patient and is confirmed by laboratory testing. Symptoms most often begin 12 to 14 days after infection, and include high fever, severe malaise, and exhaustion with headache and backache.
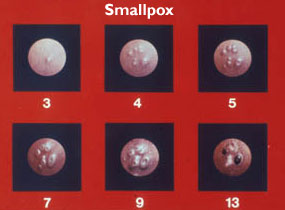
Source: WHO Smallpox slide set as a PDF
A rash of small, pink bumps appears in the mouth, on the face and forearms, and then spreads to the trunk of the body and legs, including the palms and soles. Within 1 to 2 days, the rash becomes first fluid-filled and then pus-filled. Lesions are round, hard and deep; they begin to crust over on day 8 or 9. A person infected with smallpox is thought to be contagious from the time the rash appears to the time it heals (~2 weeks). Survivors are often left disfigured, with permanent scars on the face.
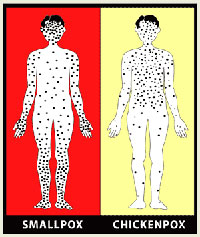
Source: CDC
Complications of smallpox include bacterial superinfections of the skin and organs, pneumonia, sepsis, arthritis, keratitis, and encephalitis. In contrast to smallpox, chickenpox (the disease with which it is most likely to be confused) is associated with a milder prodromal illness and a rash that is more superficial, usually starts on the trunk and spreads centripetally, has lesions in different stages of evolution, and rarely appears on the palms or soles.
Smallpox is caused by 1 of 2 closely related strains: variola major and variola minor. The 2 viruses are indistinguishable except by polymerase chain reaction (PCR) testing. Variola minor infection (known as Alastrim) causes fewer systemic symptoms, a less extensive rash, less scarring, and fewer fatalities.
During the era of naturally occurring smallpox, several variations of variola major disease were recognized. See Table 1.
|
Table I: WHO classification of smallpox types, based on a study of 3,544 patients in India, 1972* |
||
| Type of variola major | Characteristics | Case-fatality rate |
| Ordinary | Most common form; 90% of cases in unvaccinated persons | 30% |
| Modified |
Milder form Produces fewer, smaller, and more superficial lesions 2% of cases in unvaccinated; 25% of cases in vaccinated persons |
Cases of modified smallpox were rarely fatal. |
| Malignant or flat |
Lesions were flatter, evolved more slowly and coalesced 7% of cases in unvaccinated persons |
97% |
| Hemorrhagic |
Difficult to diagnose; rash accompanied by bleeding into mucous membranes and skin Less than 3% of cases |
Near 100% |
| Variola sine eruptione (without rash) |
Occurred in previously vaccinated contacts or in infants with maternal antibodies Affected persons were asymptomatic or had a short-lived fever, headache, and influenza-like symptoms Transmission of clinical smallpox had not been documented for variola sine eruptione |
Not available; however, in cases of variola minor, death occurred in <1% of persons. |
Derived from Fenner F, Henderson DA, Arita I, Ježek Z, and Ladnyi ID. Smallpox and Its Eradication. Geneva: World Health Organization; 1988. Page 4.
Post-exposure vaccination within 1 to 3 days after exposure to variola can prevent the clinical manifestations of smallpox. Vaccination within 4 to 7 days post-exposure can reduce the severity of disease.
There is no currently licensed antiviral treatment for smallpox. The protease inhibitor SIGA-246 (SIGA Technologies, Inc.) was awarded orphan drug status by the FDA in December 2006 and is currently undergoing clinical trials.
Antibiotics may help treat secondary bacterial infections, and supportive therapy might also benefit patients.
ACAM2000 (Sanofi) is the licensed vaccine for smallpox, currently in the U.S. Strategic National Stockpile (SNS). This vaccine contains live vaccinia virus, a relative of variola. On rare occasions, immunization with vaccinia vaccines can cause serious and potentially life-threatening reactions. Because of the potential for these reactions, newer vaccines are being pursued.
The SNS contains enough smallpox vaccine to immunize every person in the U.S.
The U.S. government does not currently recommend smallpox vaccination for the general public. In December 2002 the president announced the Smallpox Vaccine Program, to vaccinate voluntarily those persons deemed to be at highest risk should a smallpox outbreak occur, including first responders and healthcare workers serving on "smallpox response teams." In all, some 40,000 civilian employees were immunized in the first few months after the program was announced, but further vaccinations are not currently being promoted. Deployed military personnel continue to be vaccinated as well as laboratory staff engaged in work with vaccinia and related viruses.
Since smallpox no longer occurs naturally, a single confirmed case is considered an emergency and will prompt implementation of federal and state smallpox response plans for surveillance and containment.
Last reviewed February 26, 2014