Our publications keep professionals working across the public, private, and academic sectors informed on the most important developments and issues in health security and biosecurity.
Find an article or report by keywords:
UPMC Center for Health Security, 2014
Anthrax is currently considered one of the most serious bioterrorism threats. In the second half of the 20th Century, several countries used B. anthracis in their biological weapons (BW) programs, and autonomous groups have also demonstrated the intent to use the bacterium in acts of terrorism.
Anthrax is a disease caused by the bacterium Bacillus anthracis. This bacterium exists in nature in 2 forms: as an active growing cell (called the vegetative form) or as a dormant spore. The spores are very hardy and tolerant to extremes of temperature, humidity, and ultraviolet light. They can survive for long periods of time (even decades) in the environment without nutrients or water. When a spore enters a mammal host, the internal environment of the host-rich in water, sugars, and amino acids-induces that spore to germinate into a vegetative cell that leads to disease.
In nature, anthrax primarily affects herbivorous mammals such as cattle, sheep, and goats.1 According to the World Health Organization (WHO), anthrax is enzootic in animal populations in much of sub-Saharan Africa and Asia as well as in some southern European countries, parts of the Americas, and some regions in Australia. Outbreaks in animals also occur sporadically in other countries around the world. Human cases of anthrax are much less frequent.2
There are 4 forms of naturally occurring human anthrax infection:
Anthrax is currently considered one of the most serious bioterrorism threats. Beginning in the second half of the 20thCentury, B. anthracis was developed by several countries as part of their biological weapons (BW) programs. Autonomous groups have also demonstrated intent to use B. anthracis in acts of terrorism. For example, as evidenced in a March 10, 2007, Department of Defense transcript of the Tribunal Hearing of Khalid Sheikh Muhammad, al Qaeda leadership has shown interest in and has worked to develop anthrax and other biological weapons.4 In 1993, the Japanese cult Aum Shinrikyo sprayed aerosols containing B. anthracis several times in attempted terrorist attacks in Tokyo.
Fortunately, the material used turned out to be ineffective, and consequently no one was sickened.5 Most notably, in October 2001, anthrax attacks were perpetrated in the US via the mail, when 7 envelopes containing B. anthracis spores were sent through the US postal system (4 were recovered). Twenty-two cases of anthrax resulted (11 inhalational, 11 cutaneous), and 5 people died from inhalational anthrax. In 2009, the FBI closed its investigation into the origin of the attacks concluding that Dr. Bruce Ivins, an anthrax researcher at US Army Medical Research Institute of Infectious Diseases had perpetrated the attack.6 However, Dr. Ivins committed suicide before charges could be filed, and the case was never tried. Other organizations have questioned the FBI's conclusion.7
Several factors contribute to concern about the potential use of B. anthracis as a biological weapon:
An analysis in 1993 by the Office of Technology Assessment of the US Congress estimated that 130,000 to 3 million deaths could occur following the release of 100 kilograms of aerosolized B. anthracis over Washington, DC, making such an attack as lethal as a hydrogen bomb.8 (See "The History of Bioterrorism: Anthrax," a short video from the US CDC.)
The diagnosis of naturally occurring anthrax should be considered if there are symptoms and signs consistent with the disease and a history of contact with sick animals or animal skins, or travel to an area where anthrax is endemic. The expected hallmark of the use of an anthrax weapon would be a sudden surge of patients presenting with symptoms of severe pneumonia and sepsis. The physical signs of cutaneous anthrax are often quite characteristic if the clinician is attuned of them. The radiographic signs of inhalational anthrax may also be quite distinct in a patient with a clinical presentation consistent with the diagnosis.
B. anthracis grows quickly and easily in routine culture. In addition, there are a number of rapid diagnostic tests for identifying anthrax at reference laboratories, but none is available widely in ordinary hospital laboratories.1
Humans may contract anthrax following contact with infected animals or contaminated animal products or by breathing in aerosolized spores. Anthrax is not transmitted from person to person.1
Because anthrax is not passed from person to person, it is not necessary to take airborne or droplet precautions when in close proximity to an infected individual, and there is no need to provide prophylaxis to close contacts of an infected patient.1
Early antibiotic treatment of anthrax is vital, as delay decreases a victim's chance for survival. Although ciprofloxacin, levofloxacin, doxycycline, and penicillin are currently the only FDA-approved antibiotics for the treatment of anthrax, other antibiotics would be effective as well.8 In an emergency, public health authorities will make recommendations for treatment based on laboratory susceptibility testing. A regimen containing ciprofloxacin, meropenem, and linezolid is recommended as first line treatment until susceptibility information is available and the possibility of meningitis has been excluded.9 Antibiotic therapy should be coupled with treatment with raxibacumab, a monocolonal antibody that targets an anthrax antigen.9 Naturally occurring cutaneous anthrax is typically treated with antibiotics for 7 to 10 days. However, in an aerosol bioterrorism attack, patients with cutaneous anthrax may also have had an inhalational exposure that could lead to coincident inhalational anthrax; therefore, it is recommended that patients with either cutaneous or inhalational anthrax in the setting of bioterrorism continue antibiotic therapy for 60 days.10
|
Clinical Presentation of Anthrax1 |
|||
|
Anthrax Infection |
Incubation Period |
Signs and Symptoms |
Lethality |
|
Inhalational |
Ranges from as little as 2 days following exposure to spores to as long as 6 to 8 weeks after exposure |
Initial symptoms are fever, headache, and muscle aches. If untreated, the disease progresses to shortness of breath, chest discomfort, shock, and death. Meningitis may complicate the clinical course. |
Historical data suggest that the case fatality rate of untreated inhalational anthrax may be as high as 90%. With appropriate treatment, a fatality rate of approximately 50% or less may be expected. |
|
Cutaneous |
Range of 1 to 12 days following exposure; incubation period is typically closer to 1 day |
The first symptom is a small sore at the point of infection that develops into a blister and later into a painless ulcer covered by a black scab. Often there is marked swelling around the ulcer. |
Approximately 20% of persons with cutaneous anthrax may die if not treated with appropriate antibiotics. With appropriate antibiotic treatment, the death rate is approximately 1%. |
|
Gastrointestinal |
Typically 1 to 6 days following exposure |
Oropharyngeal: Symptoms are fever, ulcers in the back of the mouth and throat, severe sore throat, difficulty swallowing, and lymph node and neck swelling. |
Without antibiotic treatment, gastrointestinal anthrax results in the death of more than 40% of affected persons.11 |
|
Injection related |
1-2 days after injection |
Inflammation or abscess at the injection site sometimes progressing to cellulitis or necrotizing fasciitis. Some patients progress to sepsis without extensive local infection. |
Of reported cases, 30% of patients died despite aggressive medical therapy. |
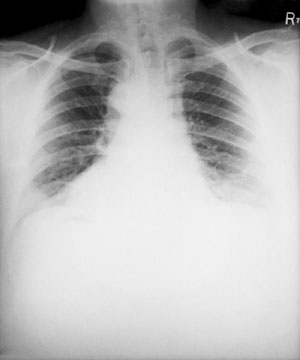
X-ray of a patient with inhalational anthrax showing bilateral effusion and widening of the mediastinum. Source: CDC Public Health Image Library (ID #5146).
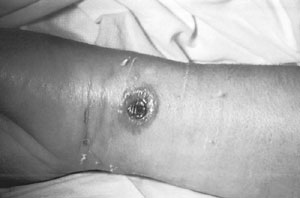
A patient with a cutaneous anthrax lesion on the arm. Source: CDC Public Health Image Library (ID #2332).
Post-exposure prophylaxis should begin immediately in persons suspected of exposure to B. anthracis spores, and should not be delayed until symptoms emerge. If susceptibility of the B. anthracis strain is unknown, initial therapy with ciprofloxacin or doxycycline is recommended for adults and children, or levofloxacin for adults. Raxibacumab can also be used for prophylaxis in special circumstances. Once started, antibiotic therapy should be continued for 60 days post-exposure. A 3-dose post-exposure anthrax vaccine regimen (at 0 weeks, 2 weeks, and 4 weeks) is recommended by the CDC, in conjunction with antibiotics.10
The greatest risk of anthrax infection occurs during the period when spores are first aerosolized (primary aerosolization). After this primary period, B. anthracis spores will settle on the ground and other surfaces, possibly in high concentrations, at which point there is a risk that B. anthracis may become airborne again (secondary aerosolization). However, the extent to which re-aerosolized spores are infectious is not known. Re-aerosolization depends on a number of variables:
Surfaces should be decontaminated to help eliminate the risk of secondary aerosolization, and this should be done in coordination with local health, public health, and environmental authorities.12
BioThrax® Anthrax Vaccine Absorbed (AVA) is produced by Emergent BioSolutions, Inc., and is the only anthrax vaccine licensed by the FDA. While it is not FDA-approved for post-exposure use, the Advisory Committee on Immunization Practices (ACIP) has issued a provisional recommendation for a 3-dose treatment in post-exposure settings under an Investigational New Drug (IND) or potentially under an emergency use authorization (EUA). AVA is a cell-free filtrate made from cultures of a strain of anthrax not capable of causing disease. A 5-dose series is required for immunization, with annual boosters.13
Anthrax Immune Globulin (AIG) antitoxin is a therapy derived from the plasma of individuals previously immunized with the anthrax vaccine. This investigational product consists of antibody directed against the anthrax protective antigen. Patients already presenting with symptoms of anthrax infection may be treated with AIG in addition to antibiotic therapy. AIG has been purchased for the CDC's Strategic National Stockpile and may be released for use in an emergency.14
Raxibacumab (ABthrax), a human monoclonal protective antigen-targeted antibody developed by Human Genome Sciences (HGS), targets anthrax toxins after they have been released by anthrax bacteria, when antibiotics might not be effective.15
Last updated February 26, 2013